Answer:
See answer below
Step-by-step explanation:
Hi there,
This question is employing the Gas Laws, specifically the relationship between internal gaseous pressure and temperature, when volume and gas quantity are constant; Gay-Lussac's Law:
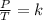 or
or
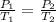
However, we will need to convert the pressures and temperatures into standard units. Pressure must be put in terms of (atm) and temperature in Kelvin (K).
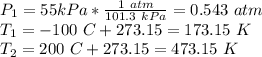
Now, solve for
 as this is what was asked for:
as this is what was asked for:
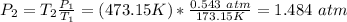
If needed, convert back into kPa, so 1.484 * 101.3 kPa = 150.3 kPa.
thanks,