Answer:
Step-by-step explanation:
Hi there,
To get started, recall the Heat-specific heat capacity equation of a substance:
Q=mCΔT
Our final temperature is 47.8 °C, since this is the way it is worded in the response (from temperature X to temperature Y)
Quite simply, we can go ahead and plug in mass, specific heat capacity, and change in temperature as all the units match up!
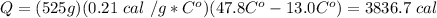
Study well and persevere.
thanks,