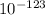Answer:
Option C: 4.54 x
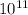 KJ/mol of nuclei
KJ/mol of nuclei
Note: Here in this question option C is not correctly put. It is 4.54 x
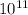 rather than 4.54 x
rather than 4.54 x
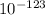 .
.
Step-by-step explanation:
If mass defect is known, then nuclear binding energy can easily be calculated, here's how:
First step is to convert that mass defect into kg.
Mass defect = 5.0446 amu
Mass defect = 5.0466 x 1.6606 x
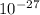
Because 1 amu = 1.6606 x
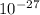 Kg.
Kg.
Mass defect = 8.383 x
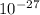 kg.
kg.
Now, we need to find out it's energy equivalent by using following equation:
Using the equation E = mc²:
where c= 3.00 x
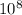 m/s²
m/s²
E = (8.383 x
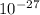 ) x (3.00 x
) x (3.00 x
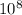 )²
)²
E = 7.54 x
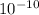 J this energy is in Joules but nuclear binding energy is usually expressed in KJ/mol of nuclei. Let's convert it:
J this energy is in Joules but nuclear binding energy is usually expressed in KJ/mol of nuclei. Let's convert it:
(7.54 x
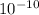 Joule/nucleus)x(1 kJ/1000 Joule)x(6.022 x
Joule/nucleus)x(1 kJ/1000 Joule)x(6.022 x
 nuclei/mol) =
nuclei/mol) =
4.54 x
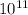 kJ/mol of nuclei .
kJ/mol of nuclei .
E = 4.54 x
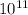 kJ/mol of nuclei . So, this is the nuclear binding energy of that atom, which is option C.
kJ/mol of nuclei . So, this is the nuclear binding energy of that atom, which is option C.
Note: Here in this question option C is not correctly put. It is 4.54 x
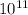 rather than 4.54 x
rather than 4.54 x