Answer:
Half-life time is 6s
Step-by-step explanation:
A first order reaction follows the formula:
ln[A] = ln[A]₀ - kt
Thus, the graph of ln[A] vs t will have as slope -k.
Graphing the given values you will obtain the linear formula:
y = -0.1154x + 0.1994
R² = 0.9997
Thus, slope is -0.1154s⁻¹ and k is:
-k = -0.1154s⁻¹; k = 0.1154s⁻¹
Now, as half-life in a first order reaction is:
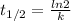
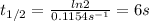
Half-life time is 6s