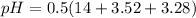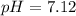Complete Question
The complete question is shown on the first uploaded image
Answer:
1) The ionic equation is
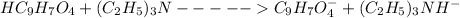
2
At equilibrium The aniline will be favored
3
The pH of the solution is
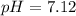
Step-by-step explanation:
From the question we are told that
The concentration of aspirin is
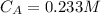
The acid dissociation constant for aspirin is
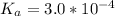
The base dissociation constant for aniline is
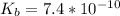
The molecular formula for aspirin is
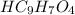
The molecular formula for aniline is
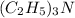
So the net ionic reaction is
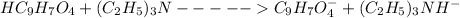
Generally pKa is mathematically evaluated as
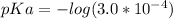
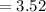
Generally pKb is mathematically represented as
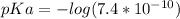
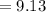
Generally
pKa + pKb = 14
So for the triethylammonium salt produced the pKa is
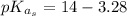
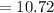
As a result of the pKa of aspirin being lower that that of triethylammonium salt at equilibrium it implies that aniline would be favored
The pH of the solution is mathematically represented as
Since they are of equal mole
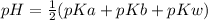
Where pKw is the pKw of water which has a value of 14