Answer: Equilibrium temperature they reach is 383 K
Step-by-step explanation:
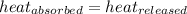
As we know that,
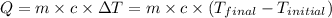
![m_1* c_1* (T_(final)-T_1)=-[m_2* c_2* (T_(final)-T_2)]](https://img.qammunity.org/2021/formulas/chemistry/college/8mq914tycmkoswztk5p5my04anxw73dmzg.png) .................(1)
.................(1)
where,
q = heat absorbed or released
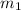 = mass of gold = 1.3 kg
= mass of gold = 1.3 kg
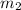 = mass of copper = 2.1 kg
= mass of copper = 2.1 kg
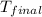 = final temperature = ?
= final temperature = ?
 = temperature of gold= 300 K
= temperature of gold= 300 K
 = temperature of copper = 400 K
= temperature of copper = 400 K
 = specific heat of gold =
= specific heat of gold =
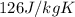
 = specific heat of copper =
= specific heat of copper =
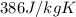
Now put all the given values in equation (1), we get
![1.3* 126* (T_(final)-300)=-[2.1* 386* (T_(final)-400)]](https://img.qammunity.org/2021/formulas/engineering/college/es4maoa2a20y3tkz6y2h03e1e2sg6koash.png)
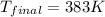
Thus equilibrium temperature they reach is 383 K