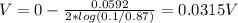Answer:
In compartment A, the solution will be concentrated with respect to compartment B, however, over time both compartments will have the same concentration. In compartment B it houses the cathode.
the voltage of the cell is 0.0315 V
Step-by-step explanation:
Given:
Electrolyte in compartment A is 0.10 M
Electrolyte in compartment B is 0.87 M
Questions:
Which half-cell houses the cathode?
What is the voltage of the cell, V = ?
In both compartments, the reactions are:
A: Sn → Sn²⁺ + 2e⁻
B: Sn²⁺ + 2e⁻ → Sn
In compartment A, the solution will be concentrated with respect to compartment B, however, over time both compartments will have the same concentration. In compartment B it houses the cathode.
The voltage of the cell
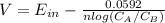
Here
n = 2 due the two electrons transferred
Ein = 0
Substituting values: