Answer:
Option C. The concentration of base is higher than the concentration of the acid.
Step-by-step explanation:
The reaction between a strong acid and a strong base follows the equation:
HA + OH⁻ ⇆ A⁻ + H₂O
The pH is:
![pH = -log [H_3O^(+)]](https://img.qammunity.org/2021/formulas/chemistry/college/v82s6122htyv5te2bu13mcu953wwumd75m.png)
If we have 100 mL of a strong acid and 100 mL of a strong base, for the pH to be more than 7, that means that the concentration of the base is higher than the concentration of the acid. This is because, the number of moles that remains in the solution after the reaction between the acid and the base will be the moles of the base. The number of moles of the reaction above is:
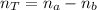 (1)
(1)
Where na: is the moles of acid, nb: the moles of the base, and nT is the total number of moles.
Case A) If the concentration of acid is the same as the concentration of the base since the volume of the acid and the base are the same, the number of moles of acid is the same as the number of moles of the base, hence, they neutralize, so the pH = 7. This is not the correct option.
Case B) If the concentration of acid is higher than the base since the volume of the acid and the base are the same, the number of moles of acid is also higher than the number of moles of the base and the total moles in equation (1) results in an excess of moles of the acid, so the pH is < 7. This is not the correct option.
Case C) If the concentration of base is higher than the concentration of the acid since the volume of the acid and the base are the same, the number of moles of the base is also higher than the number of moles of the acid and the total moles in equation (1) results in an excess of moles of the base, so the pH is > 7. This is the correct option.
Case D) The water autoionizes to give [OH-] ions. The autoionization of the water produces the same concentration of acid that the base, so this is not the correct option.
From all of the above, the correct option is C. For the pH to be more than 7, the concentration of the base is higher than the concentration of the acid.
I hope it helps you!