Answer:
The structure for the compound elucidated (4-Ethylbenzoic acid) is attached below.
Step-by-step explanation:
Degree of Unsaturation =
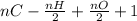 (where n = no of moles)
(where n = no of moles)
=
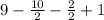
= 9 - 6+1 = 4
⇒ 2 ring. 3 double bonds.
From the 1H NMR data given (peaks labeled A-E)
A. 1.2 (3H, t) ⇒ -CH3
B. 2.6 (2H, q) ⇒ -C=C-H or Aromatic-CH3
C. 7.3 (2H, d) ⇒ Aro.-H
D. 8.0 (2H, d) ⇒ Aro.-H
E. 11.0 (1H, s) ⇒ -COOH