Answer:
0.0693M Fe
Step-by-step explanation:
It is possible to quantify Fe in a sample using Mn as internal standard using response factor formula:
F = A(analyte)×C(std) / A(std)×C(analyte) (1)
Where A is area of analyte and std, and C is concentration.
Replacing with first values:
F = 1.05×2.00mg/mL / 1.00×2.50mg/mL
F = 0.84
In the unknown solution, concentration of Mn is:
13.5mg/mL × (1.00mL/6.00mL) = 2.25 mg Mn/mL
Replacing in (1) with absorbances values and F value:
0.84 = 0.185×2.25mg/mL / 0.128×C(analyte)
C(analyte) = 3.87 mg Fe / mL
As molarity is moles of solute (Fe) per liter of solution:
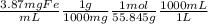 = 0.0693M Fe
= 0.0693M Fe