Answer: The reaction will produce 0.0003 g of solid silicone.
Step-by-step explanation: Use Ideal Gas Law to determine how many mols of SiH4 there are in the reaction. The formula for the Law is:
PV = nRT
where:
P is pressure in atm
V is volume in L
n is mols
R is gas constant, R = 0.082 L.atm.mol⁻¹.K⁻¹
T is temperature in K
First, transform the variables to units that equals the constant unit:
Pressure:
Pa in atm: P =
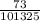 = 0.00074 atm
= 0.00074 atm
Temperature:
°C in K: T = 600 + 273.15 = 873.15 K
With those values, determine the mols (n):
PV = nRT
n =
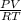
n =
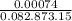
n = 0.00000103 mols
From the balanced reactio equation, 1 mol of silane produces 1 mols of solid silicone, so, 0.00000103 mols of silane produces 0.00000103 mol of solid silicone.
To find the mass in grams:
Si has molar mass of 28.0855g/mol
1 mol = 28.0855 g
0.00000103 mols = m
m = 28.0855*0.00000103
m = 0.0003 grams
It will be produced 0.0003 grams of solid silicone