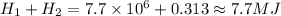Answer:
7.7 MJ
Step-by-step explanation:
Let water specific heat be c = 0.004186 J/kgC and specific latent heat of vaporization be L = 2264705 J/kg
There would be 2 kinds of heat to achieve this:
- Heat to change water temperature from 78C to boiling point:
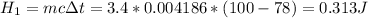
- Heat to turn liquid water into steam:
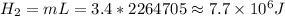 or 7.7 MJ
or 7.7 MJ
So the total heat it would take is