Answer: The concentration of the diluted solution is 0.635 M.
Step-by-step explanation:
The given data is as follows.
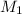 = 12.7 M,
= 12.7 M,
 = 25.0 ml = 0.025 L (as 1 ml = 0.001 L)
= 25.0 ml = 0.025 L (as 1 ml = 0.001 L)
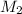 = ? ,
= ? ,
 = 0.5 L
= 0.5 L
Therefore, we will calculate the molarity of the solution as follows.
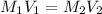
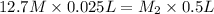
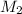 = 0.635 M
= 0.635 M
Thus, we can conclude that the concentration of the diluted solution is 0.635 M.