Answer:
a) pH of methylamine = 11.91
b) Volume of milliliters of titrant required to reach equivalence point = 36.59 mL
c) The pH at equivalence point = 5.92
Step-by-step explanation:
a)
Before the titrant is added; the value for pH of the methylamine is calculated as:
![pOH = (1)/(2)[pK_b \ - \ log \ C]](https://img.qammunity.org/2021/formulas/chemistry/college/qolfwdh5t5n7kp0ta2l3cqol2xl7ne3eh4.png)
where ;
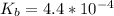
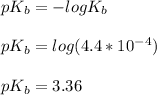
![pOH = (1)/(2)[pK_b \ - \ log \ C]](https://img.qammunity.org/2021/formulas/chemistry/college/qolfwdh5t5n7kp0ta2l3cqol2xl7ne3eh4.png)
![pOH = (1)/(2)[3.36\ - \ log \0.15]](https://img.qammunity.org/2021/formulas/chemistry/college/yu4xcycbsu97jmmzwk782vzrzj0q1fcoix.png)
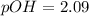
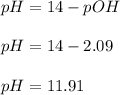
b)
How many milliliters of titrant are required to reach the equivalence point?
Millimoles of base = (25.00 mL × 0.1500 M) of methylamine = 3.75
3.75 millimoles of HCl is required to reach equivalence point.
3.75 = Volume × 0.1025
Volume of milliliters of titrant required to reach equivalence point = 36.59 mL
c)
The total volume = 36.58 + 25.00 = 61.58 mL
Concentration of the salt; i.e [salt] =
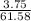
[salt] = 0.061 M
![pOH = (1)/(2)[pK_w+pK_b+log \ C]](https://img.qammunity.org/2021/formulas/chemistry/college/6ucey2bli7sengcztbt8hiamv190egdm5k.png)
![pOH = (1)/(2)[14+3.36+log \ 0.061]](https://img.qammunity.org/2021/formulas/chemistry/college/4r5l5y3nm17iopzcb3cd7q1cf2cfs4lzqw.png)
![pOH = (1)/(2)[16.15]](https://img.qammunity.org/2021/formulas/chemistry/college/x9vw1jm26absnff35njt1majtch0m31wn0.png)
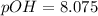
pOH ≅ 8.08
pH = 14 - 8.08
pH = 5.92