Answer:
the answe is 40.3775 L
Step-by-step explanation:
from boyles law for ideal gas,
we know,
Pressure(P)*Volume(V)= Constant [provided Temperature and the mass is uniform]
then
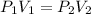
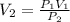 ............1
............1
now, P1= 1.55 atm
V1=52.1 L
P2=2 atm
V2=?
we can find the value of V2 or changed volume of the gas by the help of equation 1.
And the answe is 40.3775 L.