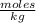Answer:
The molality is 4.6
Step-by-step explanation:
Molality (m) is a concentration measurement that indicates the number of moles of solute that are dissolved in 1 kilogram of solvent.
The Molality of a solution is determined by the following expression:
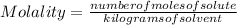
The molality is expressed in units (
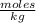 ).
).
In this case:
- number of moles of solute: 2.3 moles
- kilograms of solvent: being 1 kg=1000 g, 500 g=0.5 kg
Replacing:

Solving:
Molality=4.6
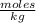
The molality is 4.6