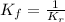Answer: The relation between the forward and reverse reaction is
Step-by-step explanation:
For the given chemical equation:
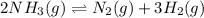
The expression of equilibrium constant for above equation follows:
![K_f=([N_2][H_2]^3)/([NH_3]^2)](https://img.qammunity.org/2021/formulas/chemistry/high-school/juf1yfp636eyjgj80kk78gxu8dvllol0ik.png) .......(1)
.......(1)
The reverse equation follows:
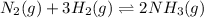
The expression of equilibrium constant for above equation follows:
![K_r=([NH_3]^2)/([N_2][H_2]^3)](https://img.qammunity.org/2021/formulas/chemistry/high-school/rj8makwr1fipgrf0xkeo8vqrac53hhfzfe.png) .......(2)
.......(2)
Relation expression 1 and expression 2, we get:
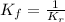
Hence, the relation between the forward and reverse reaction is