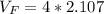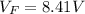Answer:
The voltage would be
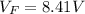
Step-by-step explanation:
From the question we are told that
The wavelength is
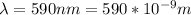
Generally when a electron changes state to a higher state an energy(E) is given off and this is equivalent to eV
So the voltage of the electron can be evaluated as
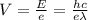
Where h is the planks constant with a constant value
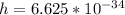
c is the speed of light
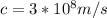
e is the value charge in one electron
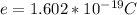
Substituting value
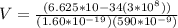
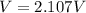
So the fourth current drop would be recorded when electron has change state four times and the voltage at this point can be mathematically evaluated as