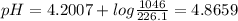Answer:
The answer is 4.8659
Step-by-step explanation:
Given:
[C₆H₅COOH]=0.11M
[C₆H₅COO]=2*0.2=0.4M
Ka=6.3x10⁻⁵
First, calculate the pKa:
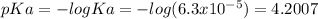
The pH is:
![pH=pKa+log(C6H5COO])/([C6H5COOH]) =4.2007+log(0.4)/(0.11) =4.7614](https://img.qammunity.org/2021/formulas/chemistry/college/hsee8dumqd44609o5l6bj6epappedve5if.png)
Like the volume is 5L, the volume of C₆H₅COO is x, then, the volume of C₆H₅COOH is 5-x
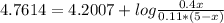
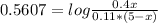
Solving for x:
x=2.49L=2490mL of C₆H₅COO
2510mL of C₆H₅COOH
The milimoles of C₆H₅COOH and C₆H₅COO is:
nC₆H₅COOH=(0.11*2510)-50=226.1mmol
nC₆H₅COO=(0.4*2490)+50=1046mmol
The pH is: