Answer:
It would take 27.5 minutes the element to decay to 154 grams.
Explanation:
The decay equation:
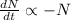
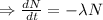
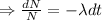
Integrating both sides
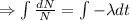
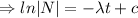
When t=0, N=
 = initial amount
= initial amount
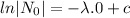
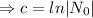
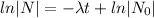
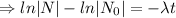
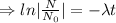
Decay equation:
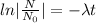
Given that, the half life of of element X is 11 minutes.
For half life,
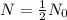 , t= 11 min.
, t= 11 min.
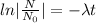
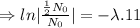
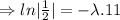
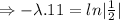
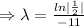
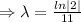 [
[
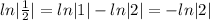 , since ln|1|=0]
, since ln|1|=0]
N=154 grams,
 = 870 grams, t=?
= 870 grams, t=?
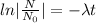
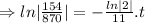
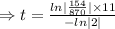
=27.5 minutes
It would take 27.5 minutes the element to decay to 154 grams.