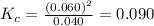Answer:
Value of
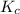 is 0.090.
is 0.090.
Step-by-step explanation:
Initial molarity of
 =
=
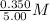 = 0.0700 M
= 0.0700 M
Construct an ICE table corresponding to the combustion reaction of carbon to determine
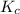
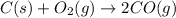
I (M): - 0.0700 0
C (M): - -x +2x
E (M): - 0.0700-x 2x
So,
![K_(c)=([CO]^(2))/([O_(2)])](https://img.qammunity.org/2021/formulas/chemistry/college/cbccu3mh43a6ydjdxmiwhm792x5pec1p5e.png) , where [CO] and
, where [CO] and
![[O_(2)]](https://img.qammunity.org/2021/formulas/chemistry/college/ojv47oslb7b2o5kruf399hwme63c5m15gm.png) represents equilibrium concentration of CO and
represents equilibrium concentration of CO and
 respectively.
respectively.
Here,
![[CO]=2x=0.060](https://img.qammunity.org/2021/formulas/chemistry/college/yy004gyejbmmew8arf6gjk7l3b85ki5mia.png)
⇒x = 0.030
So,
![[O_(2)]](https://img.qammunity.org/2021/formulas/chemistry/college/ojv47oslb7b2o5kruf399hwme63c5m15gm.png) = 0.0700-x = (0.0700-0.030) = 0.040
= 0.0700-x = (0.0700-0.030) = 0.040
Hence,