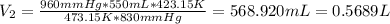Answer:
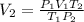
And replacing the info we have:
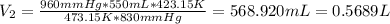
Explanation:
For this case we wan assume that we have an ideal gas and we can use this relation:
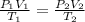
And for this case we know:
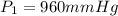
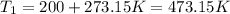
Becuase
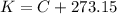
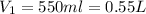
Because 1L = 1000 mL
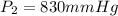
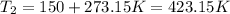
And if we solve for V2 we got:
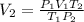
And replacing the info we have: