Answer : The number of grams of calcium perchlorate is, 0.00253 grams.
Explanation :
Molar mass of calcium perchlorate = 238.9 g/mol
As,
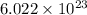 formula units present in 238.9 g of calcium perchlorate
formula units present in 238.9 g of calcium perchlorate
So,
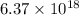 formula units present in
formula units present in
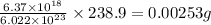 of calcium perchlorate
of calcium perchlorate
Therefore, the number of grams of calcium perchlorate is, 0.00253 grams.