Answer:
The scroll is 1949 years old, thus the archeologists are right.
Explanation:
The decay equation of ¹⁴C is:
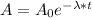 (1)
(1)
Where:
A₀: is the initial activity
A: is the activity after a time t = 79%*A₀
λ: is the decay rate
The decay rate is:
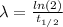 (2)
(2)
Where
 : is the half-life of ¹⁴C = 5730 y
: is the half-life of ¹⁴C = 5730 y
By entering equation (2) into equation (1) we can find the age of the scrolls.
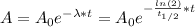
Since, A = 79%*A₀, we have:
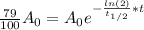
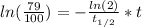
Solving the above equation for t:
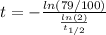
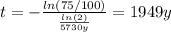
Hence, the scroll is 1949 years old, thus the archeologists are right.
I hope it helps you!