Answer:
The amount of heat is 84.4894kJ
Step-by-step explanation:
Given data:
mass of octane=148g
The moles of octane is:
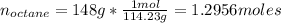
The melting point is=-56.82°C
ΔHfus=20.73kJ/mol
The heat of fusion is:
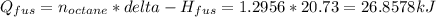
The heat to bring the octane to a temperature of 117.8°C is:
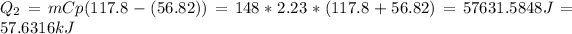
The total heat in the process is:
Qtotal=Qfus+Q₂=26.8578+57.6316=84.4894kJ