Answer:
Metallic.
Step-by-step explanation:
Hello,
In this case, when nickel and tin bond, the difference in their electronegativities results:
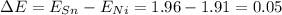
Such difference, in addition to the fact that nickel is a transition metal and tin a metal, will suggest that the predominant type of bond for this substance is metallic as the attractive force between the conduction of electrons and positively charged metal ions is present.
Best regards.