Answer:
a) 2*10^14 electrons
Step-by-step explanation:
To find the amount of electrons you take into account that:
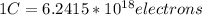
by replacing this values in the factor C of the charge given by the exercise you obtain:
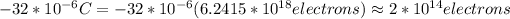
= 2*10*10^13 =20*10^13 electrons (you take the absolute value)
hence, the total number of electrons in a charge of 32*10^-6 C is 2*10^13 electrons