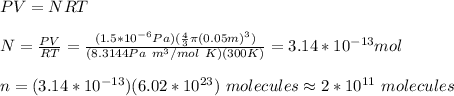Answer:
a. 9947 m
b. 99476 times
c. 2*10^11 molecules
Step-by-step explanation:
a) To find the mean free path of the air molecules you use the following formula:
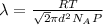
R: ideal gas constant = 8.3144 Pam^3/mol K
P: pressure = 1.5*10^{-6} Pa
T: temperature = 300K
N_A: Avogadros' constant = 2.022*10^{23}molecules/mol
d: diameter of the particle = 0.25nm=0.25*10^-9m
By replacing all these values you obtain:
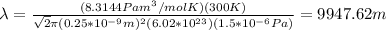
b) If we assume that the molecule, at the average, is at the center of the chamber, the times the molecule will collide is:
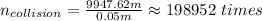
c) By using the equation of the ideal gases you obtain: