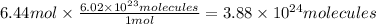Answer:
3.88 × 10²⁴ molecules
Step-by-step explanation:
In order to solve this question, we need to consider the Avogadro's number. know that 1 mole of particles contains 6.02 × 10²³ particles. This applies to different kinds of particles: atoms, molecules, electrons.
In this case, 1 mole of molecules of oxygen gas contains 6.02 × 10²³ molecules of oxygen gas. We will use this relation to find the number of molecules of oxygen gas in 6.44 moles of oxygen gas.