Answer:
a)
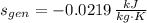 , b) No.
, b) No.
Step-by-step explanation:
The turbine is modelled after the Second Law of Thermodynamics, which states:
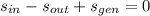
The entropy generation per unit mass is:
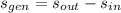
The specific entropy for steam at entrance and exits are obtained from property tables:
Inlet (Superheated Steam)
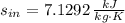
Outlet (Liquid-Vapor Mixture)
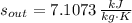
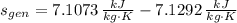
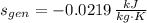
b) It is not possible, as it contradicts the Kelvin-Planck and Claussius Statements, of which is inferred that entropy generation can only be zero or positive.