Answer: salt
Step-by-step explanation:
Arrhenius acids are substances which dissociate in water to give
 ions.
ions.
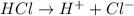
Arrhenius bases are substances which dissociate in water to give
 ions.
ions.
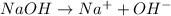
Thus when they combine, they neutralize each other and produce salt and water.
