Answer: The volume occupied by the gas is 5295.1 L
Step-by-step explanation:
To calculate the number of moles, we use the equation:

Given mass of hydrogen gas = 454.5 g
Molar mass of hydrogen gas = 2 g/mol
Putting values in above equation, we get:
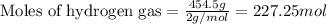
To calculate the volume, we use the equation given by ideal gas equation:
PV=nRT
where,
P = pressure of the gas = 1.050 at m
V = Volume of gas = ?
n = number of moles of gas = 227.25 moles
R = Gas constant =
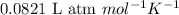
T = temperature of the gas =
![25^oC=[25+273]K=298K[/tex ]</p><p><strong>Putting values in above equation, we get: </strong></p><p>[tex]1.050atm* V=227.25mol* 0.0821\text{ L atm }mol^(-1)K^(-1)* 298K\\\\V=(227.25* 0.0821* 298)/(1.050)=5295.1L]()
Hence, the volume occupied by the gas is 5295.1 L