Step-by-step explanation:
Strong electrolytes are those solutions which undergo complete dissociation when dissolved in water. The dissociation of strong electrolytes is given by a right arrow.
NaCl being an ionic compound dissociate in water to give sodium and chloride ions which help in conduction of electricity.
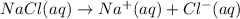
Whereas sugar being a covalent compound, do not dissociate into ions when dissolved in water and thus cannot conduct electricity.