Answer :
(a) The number of moles of D produced can be, 6.67 moles.
(b) The volume of D prepared can be, 24.5 L
Explanation :
The given chemical reaction is:
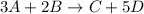
Part (a) :
From the balanced chemical reaction, we conclude that:
As, 3 moles of A react to give 5 moles of D
So, 4 moles of A react to give
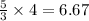 moles of D
moles of D
Thus, the number of moles of D produced can be, 6.67 moles.
Part (b) :
As we know that 1 moles of substance occupies 22.4 L volume of gas.
As,
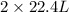 volume of B gives
volume of B gives
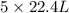 volume of D
volume of D
As, 9.8 L volume of B gives
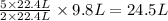 volume of D
volume of D
Thus, the volume of D prepared can be, 24.5 L