Answer:
A) As the initial concentration of H₂ is increased, the reaction rate also increases.
B) Yes, [H₂] is part of the rate law.
Step-by-step explanation:
For the reaction:
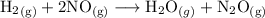
The rate law will have the form:
![\displaystyle \text{Rate} = k\left[\text{H$_2$}\right]^n\left[\text{NO}\right]^m](https://img.qammunity.org/2023/formulas/chemistry/college/q7j0fl7x9t2jgvvw7nys31u9nq34y2s3ur.png)
Where n and m are the respective order of reactions.
From the table, we can see that as the concentration of H₂ increases, the instantaneous rate also increases.
Therefore, [H₂] is indeed part of the rate law. If it isn't, changing its concentration will have no effect on the instantaneous rate of the reaction.