Answer:
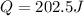
Step-by-step explanation:
Hello,
In this case, the increase in the temperature involves the addition of heat that is defined in terms of mass, heat capacity and temperature:
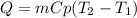
In this case, the heat capacity of iron is 0.450 J/(g*K), thus the heat results:
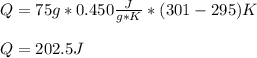
In such a way, since the temperature is increased heat is added, that is why it is positive.
Best regards.