Hi, the question has some missing part. The complete question should be " If the
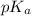 of
of
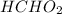 is 3.74 and the pH of an
is 3.74 and the pH of an
 solution is 3.89, it means that -
solution is 3.89, it means that -
(a)
![[HCHO_(2)]=[NaCHO_(2)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/d81onkh1jd11n6qozz09u3icr0dmgjyls0.png)
(b)
![[HCHO_(2)]> [NaCHO_(2)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/3rmuo81qfql3n84sxse4hdud9597f1wlb2.png)
(c)
![[HCHO_(2)]< [NaCHO_(2)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/ddl1ao5jlueba4235vpj34kkerb1tdz1zj.png)
Which of the options are true?"
Answer: Option (c) is true.
Step-by-step explanation:
 is an weak acid-conjugate base buffer. Hence, in accordance with Henderson-Hasselbalch equation-
is an weak acid-conjugate base buffer. Hence, in accordance with Henderson-Hasselbalch equation-
![pH=pK_(a)(HCHO_(2))+log(([CHO_(2)^(-)])/([HCHO_(2)]))](https://img.qammunity.org/2021/formulas/chemistry/high-school/makfk5dl0u9fw75v17e81ag85xugup332k.png)
Here, pH = 3.89,
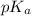 of
of
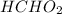 = 3.74
= 3.74
So,
![3.89=3.74+log(([CHO_(2)^(-)])/([HCHO_(2)]))](https://img.qammunity.org/2021/formulas/chemistry/high-school/dqn9cgrzpn1vos8gqievdq2jzf9096xgks.png)
or,
![([CHO_(2)^(-)])/([HCHO_(2)])](https://img.qammunity.org/2021/formulas/chemistry/high-school/kqpe6y5d714tnnn25xntncjhx3v2ywiabo.png) = 1.41 (>1)
= 1.41 (>1)
So,
![[CHO_(2)^(-)]> [HCHO_(2)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/twk5tjhyhg5c0j1g6mrzlv84zoiols66ly.png)
or,
![[NaCHO_(2)]> [HCHO_(2)]](https://img.qammunity.org/2021/formulas/chemistry/high-school/fdkx9xl320od9bbbjffyz4lekd6xo9jf0b.png)
Hence, option (c) is correct.