Answer:
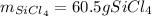
Step-by-step explanation:
Hello,
In this case, the undergoing balanced chemical reaction is:
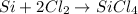
Thus, we compute the available moles of silicon and the reacted moles of silicon with the 60.0 grams of chlorine gas as shown below, in order to identify the limiting reactant and subsequently the actual moles that are effectively reacting:
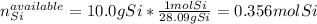
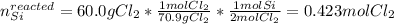
In such a way, since there are less available moles of silicon, we conclude silicon is the limiting reactant, for that reason, the yielded mass of silicon tetrachloride turns out:
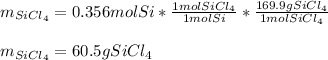
Best regards.