Answer: The reaction is spontaneous and there is not enough information to calculate the cell voltage.
Step-by-step explanation:
The substance having highest positive
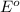 reduction potential will always get reduced and will undergo reduction reaction.
reduction potential will always get reduced and will undergo reduction reaction.
Oxidation reaction occurs at anode and reduction reaction occurs at cathode.
The half reactions for the cell occurring at cathode follows:
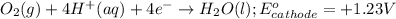
The half reactions for the cell occurring at anode follows:
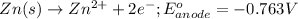 ( × 2)
( × 2)
The balanced equation for the overall reaction of the cell follows:
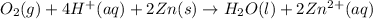
For the reaction to be spontaneous, the Gibbs free energy of the reaction must come out to be negative.
Relationship between standard Gibbs free energy and standard electrode potential follows:
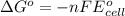
For a reaction to be spontaneous, the standard electrode potential must be positive.
To calculate the
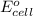 of the reaction, we use the equation:
of the reaction, we use the equation:
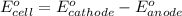
Putting values in above equation, we get:
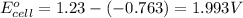
As, the standard electrode potential of the cell is coming out to be positive, the reaction is spontaneous in nature.
- To calculate the EMF of the cell, we use the Nernst equation, which is:
![E_(cell)=E^o_(cell)-(0.059)/(n)\log ([Zn^(2+)]^2)/([H^(+)]^4* p_(O_2))](https://img.qammunity.org/2021/formulas/chemistry/college/4iwq7f0t4d2l80x9tkcmwby5idr6qu7ogv.png)
As, the concentrations and partial pressures are not given. So, there is not enough information to calculate the cell voltage.
Hence, the reaction is spontaneous and there is not enough information to calculate the cell voltage.