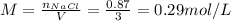Answer:
The molarity of the resulting solution is 0.29 mol/L
Step-by-step explanation:
Given:
nNaCl = moles = 0.87 mol
V = volume of solution = 3 L
Question: What is its molarity of the resulting solution, M = ?
First, you need to know that the molarity of a solution is the number of moles of the solute, in this case the salt (NaCl), divided by the volume of the solution: