Answer:
Enthalpy change for the reaction is -67716 J/mol.
Step-by-step explanation:
Number of moles of
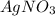 in 50.0 mL of 0.100 M of
in 50.0 mL of 0.100 M of
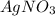
= Number of moles of HCl in 50.0 mL of 0.100 M of HCl
=
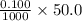 moles
moles
= 0.00500 moles
According to balanced equation, 1 mol of
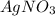 reacts with 1 mol of HCl to form 1 mol of AgCl.
reacts with 1 mol of HCl to form 1 mol of AgCl.
So, 0.00500 moles of
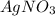 react with 0.00500 moles of HCl to form 0.00500 moles of AgCl
react with 0.00500 moles of HCl to form 0.00500 moles of AgCl
Total volume of solution = (50.0+50.0) mL = 100.0 mL
So, mass of solution = (
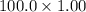 ) g = 100 g
) g = 100 g
Enthalpy change for the reaction = -(heat released during reaction)/(number of moles of AgCl formed)
=
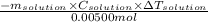
=
![\frac{-100g* 4.18\frac{J}{g.^(0)\textrm{C}}* [24.21-23.40]^(0)\textrm{C}}{0.00500mol}](https://img.qammunity.org/2021/formulas/chemistry/college/60hfjwehb9rej8xsuoehmtrbit3kfo1216.png)
= -67716 J/mol
[m = mass, c = specific heat capacity,
 = change in temperature and negative sign is included as it is an exothermic reaction]
= change in temperature and negative sign is included as it is an exothermic reaction]