Answer:
The workdone by both N₂ and neon gas is 49.3 J
The change in internal energy of N₂ and neon gas is 125.6 J and 73.54 J respectively
The heat for N₂ and neon gas is 171.9 J and 122.84 J respectively.
Step-by-step explanation:
Given that:
number of moles = 0.05 mole
mass of the piston = 30 kg
diameter = 5.00 cm = 0.05 m
Area (A) = πr²
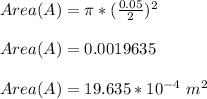
The piston is said to move from 30 cm - 40 cm
So, the change in volume ΔV is calculated as:
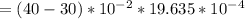

Outside the cylinder; the pressure
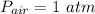 = 101325 Pa
= 101325 Pa
Thus, workdone
 = PΔV
= PΔV
=
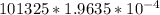
= 19.90 J
The gravitational work
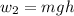
Given that the height (h) = 10 cm = 0.1 m
Then;

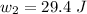
The total workdone
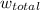 for both cases is:
for both cases is:
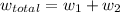
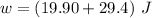
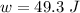
The pressure of gas inside the cylinder is determined as:
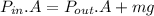
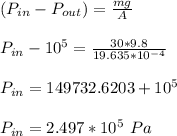
a). assuming that the gas is N₂.
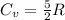
Thus, the change in internal energy ΔU is given as:
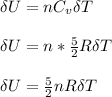
Since
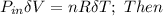 ;
;
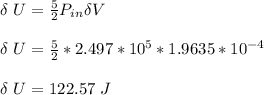
ΔU ≅ 125.6 J
The heat Q = ΔU + W
Q = (122.6 + 49.3) J
Q = 171.9 J
b) In Neon gas:
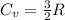
∴
change in internal energy is;
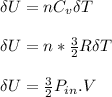
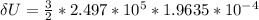
ΔU = 73.54 J
The heat Q = ΔU + W
Q = (73.54 + 49.3) J
Q = 122.84 J