Answer:
4.43L is final volume of the ballon
Step-by-step explanation:
Avogadro's law of ideal gases states that equal volumes of gases, at the same temperature and pressure, have the same number of molecules.
The formula is:
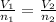
Where V and n are volume and moles of the gas in initial and final conditions.
If the initial conditions are 0.0145 moles and 2.54L and final amount of moles is 0.0253moles, final volume is:
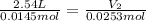
V₂ = 4.43L is final volume of the ballon