The question is incomplete, here is the complete question:
Methanol, is made by using a catalyst to react carbon monoxide with hydrogen at high temperature and pressure. Assuming that 450.0 mL of CO and 825 mL of
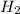 are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)
are allowed to react, answer the following questions. (Hint: First write the balanced chemical equation for this reaction.)
Which reactant is in excess?
Answer: The excess reactant in the reaction is carbon monoxide.
Step-by-step explanation:
Excess reactant is defined as the reactant which is present in large amount in a reaction. The formation of product does not depend on this reactant and some amount of it remains after the completion of the reaction.
We are given:
Mass of CO = 450.0 mL
Mass of hydrogen gas = 825 mL
The balanced chemical equation for the reaction of carbon monoxide and hydrogen gas follows:
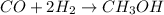
By Stoichiometry of the reaction:
2 mL of hydrogen gas reacts with 1 mL of carbon monoxide
So, 825 mL of hydrogen gas will react with =
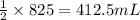 of carbon monoxide
of carbon monoxide
As, given amount of carbon monoxide is more than the required amount. So, it is considered as an excess reagent.
Hydrogen gas is considered as a limiting reagent because it limits the formation of product.
Hence, the excess reactant in the reaction is carbon monoxide.