Answer:
4*10^22 photons
Step-by-step explanation:
To find the number of photons is necessary to calculate the total energy of the light emitted in one hour = 3600s:
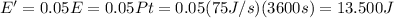
Furthermore, is necessary to find the associated energy to the photon 0f 570nm with following formula:
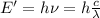
h: Planck's constant = 6.62*10^-34 Js
c: speed of light = 3*10^8 m/s
wavelength = 570*10^-9 m
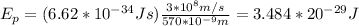
Finally you divide E' between Ep to find the number of photons:
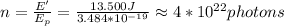
the number of emitted photons is 4*10^22