Answer:
528g AlCl3
Step-by-step explanation:
***Remember that you need all the terms except "g AlCl3" to cancel out
The atomic mass of Al is about 27g and the atomic mass of AlCl3 is about 132g
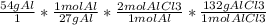
You need to take all the numbers and multiply/divide
528g AlCl3 should be your answer