Answer:
413 nm
Step-by-step explanation:
The wavelength is defined as
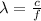
Where
 represents the wavelength,
represents the wavelength,
 the speed of the wave which is a constant and
the speed of the wave which is a constant and
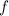 is the frequency of the wave,
is the frequency of the wave,
In this case, we know
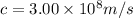
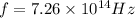 , where
, where
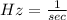
Replacing these given values in the formula, we have
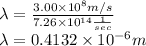
But, we know that
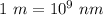
So, we use the equivalence to transform the wavelength from meters to nanometers.
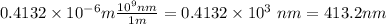
Therefore, the wavelength is 413.2 nanometers, approximately.