Answer:
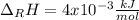
Step-by-step explanation:
Hello,
In this case, the molar enthalpy of reaction is obtained by dividing the involved energy by the reacting moles:
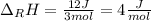
Thus, it is important to notice that the compound "uses" the energy, it means that it absorbs the energy, for that reason the sign is positive. Moreover, computing the result in kJ/mol we finally obtain:
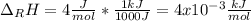
Best regards.