Answer:
F = 6.604N
Step-by-step explanation:
To find the electric force between the protons you use the Coulomb's formula:
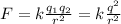
where you have used that both charges have the same charge.
K: Coulomb's constant = 8.98*10^9 Nm^2/C^2
r: distance between the charges = 5.9fm = 5.9*10^{-15}m
q: 1.6*10^{-19}C
By replacing the values of k, q and r you obtain:
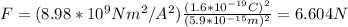
If you compare this force with the nuclear one Fn = 2000N you obtain:
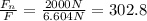
hence, the nuclear force is about 302.8 times stronger than the Coulomb's force