Answer:
787.5 grams of glucose (C₆H₁₂O₆) can prepare 1750 mL of a 2.50 M solution
Step-by-step explanation:
Molarity (M) is a concentration measure that indicates the number of moles of solute that are dissolved in a given volume.
Molarity is expressed in the following way:
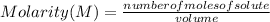
Molarity is expressed in units
 .
.
2.50 M means that in 1 L of solution there are 2.5 moles of glucose. So, you apply a rule of three as follows: if in 1 L there are 2.5 moles of glucose, in 1.75 L (1750 mL, being 1000 mL = 1 L) how many moles of the compound are there?
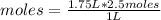
moles=4.375
Being:
- C: 12 g/mole
- H: 1 g/mole
- O: 16 g/mole
The molar mass of glucose is:
C₆H₁₂O₆= 6* 12 g/mole+ 12* 1 g/mole + 6* 16 g/mole
C₆H₁₂O₆= 180 g/mole
Then you can apply a rule of three as follows, knowing the moles in 1750 mL and the molar mass: if there are 180 g of glucose in 1 mole, how much mass is there in 4.375 moles?
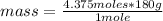
mass= 787.5 g
787.5 grams of glucose (C₆H₁₂O₆) can prepare 1750 mL of a 2.50 M solution